Author: Latrice Landry
As the U.S. and other countries rally to address the impact of the COVID-19 pandemic, genetics remains front and center. Here, we discuss four points about COVID-19 that every genetics trainee should know.
Point 1: Genetics is Central to COVID-19 Testing.
Testing is not only central to the diagnosis of patients presenting with symptoms but also to identifying asymptomatic carriers. Understanding the proportion of the population with COVID-19 immunity through testing can also help shape ‘reopening’ policies. There is no question testing is central to solving the pandemic.
The most common COVID-19 test is a reverse transcriptase polymerase chain reaction (RT-PCR) assay. For many in ASHG, RT-PCR is an old and familiar friend. Despite requiring relatively extensive time and skill to perform, RT-PCR is a common method and the equipment, reagents, and operation are familiar to most clinical molecular labs. Consequently, multiple commercial and academic labs (many led by ASHG members) launched RT-PCR-based COVID-19 molecular testing for active infection this spring. RT-PCR testing works by identifying a RNA sequence unique to Coronavirus-19 in actively infected individuals, detecting active cases early in the infectious process with a reported average specificity between 80-85%.
Beyond RT-PCR tests, many other assays are also being developed, including over 50 diagnostic tests authorized by the Food and Drug Administration. Further, there is an additional need to test for potential immunity through antibody testing. While the RT-PCR test captures active infection, its detection rate is suggested to decrease at the tenth day of infection. Therefore, the antibody test, whose performance peaks around 14 days is preferred to identify previously infected individuals or individuals in the later stages of infection. When it comes to testing for COVID-19, it is important to match the right test to the right timeline to improve accuracy, as not all tests work in all scenarios.
Point 2: Genetics is Central to COVID-19 Vaccine Development
Dr. Anthony Fauci, Director of the National Institute of Allergy and Infectious Diseases, suggested a COVID-19 vaccine could be available by early 2021. His self-acknowledged optimistic time-frame is based on a new type of vaccine. Unlike traditional whole virus vaccines that require cell line and purification procedures that complicate manufacturing, synthetic mRNA vaccines are based on reconstruction of viral genes from a known genetic blueprint. This means that mRNA vaccine development can commence as soon as the target antigen sequence is known, saving time.
It is not yet known if mRNA vaccines perform the same as whole virus vaccines but testing in animal models suggests that mRNA vaccines provide immunity against influenza, zika, rabies, and other viral illnesses. Still, many questions about creating a COVID-19mRNA vaccine remain. Would the mRNA selected for vaccine development correctly balance the number of relevant and irrelevant proteins needed for immunity? What is the antibody concentration needed for protection from COVID-19? What is the comparative quality of antibodies between vaccines? As of April 8, 2020, there were 115 vaccine candidates in various preclinical stages–hopefully we will answer these questions soon, and geneticists will be integral to doing so.
Point 3: Policy Matters.
There are many policies affecting COVID-19 testing and vaccine development. While the emergency nature of the COVID-19 pandemic has affected the speed of processes, the federal government response to COVID-19 continues to require concerted effort from multiple agencies, flexibility and patience. For instance, the designation of the crisis as a public health emergency was pivotal to the current level of test availability. Furthermore, the public health emergency allowed for wider use of emergency use authorization (EUA) to accelerate both test and vaccine development. Here we provide a snapshot of key events in the U.S. implementation of COVID-19 testing and vaccine development. An understanding of genetics was key to many of these policy decisions.
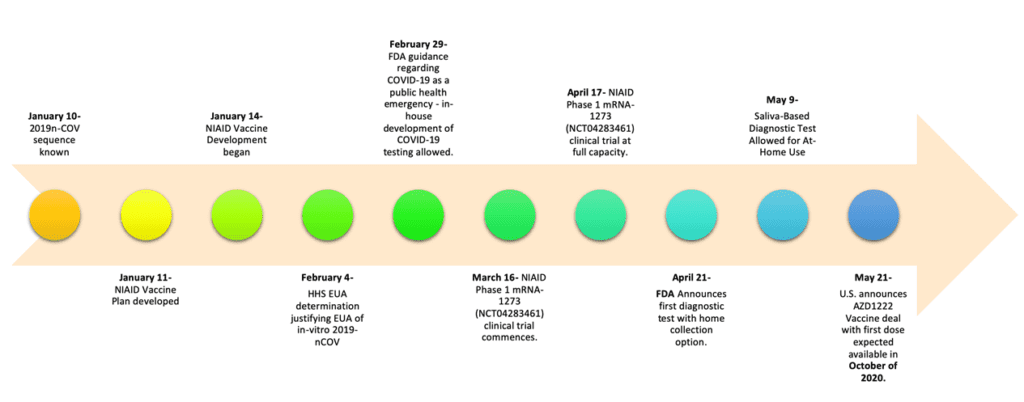
Point 4- Genetic Research is pivotal in solving the COVID-19 crisis.
The COVID-19 pandemic remains a fluid situation. We have learned a lot in a relatively short period of time. However, there is much more work to be done before the emergency will end. Our last and final point is that genetic research WILL play a pivotal role in the conclusion of this pandemic.
